Introduction: Advanced systemic mastocytosis (AdvSM) is characterized by presence of the KIT D816V mutation and variably other somatic mutations in the mast cell but also non-mast cell lineages, e.g. monocytes or eosinophils, in ≥80-90% of patients. Midostaurin is an approved multikinase/KIT inhibitor for treatment of AdvSM. The intricate landscape in assessment of response, resistance or progression, the occurrence of adverse events with its impact on dose modification and the recent approval of the specific KIT D816V inhibitor avapritinib contribute to complex decision-making. We here sought to characterize the most valuable baseline and on-treatment parameters for prediction of response and survival in midostaurin-treated patients with AdvSM.
Methods: On basis of the ´German Registry on Disorders of Eosinophils and Mast Cells' (GREM), this retrospective-prospective analysis included 79 patients with AdvSM. Treatment was initiated between 2009 and 2022 accumulating into a 239 patient-years overall follow-up period. In addition to common serological, morphological, and molecular response parameters, we specifically examined the recurrent observation of an early flare-up in alkaline phosphatase (AP) levels, which was defined as a ≥25% AP increase within three months after treatment initiation, followed by a subsequent decline below baseline within six months.
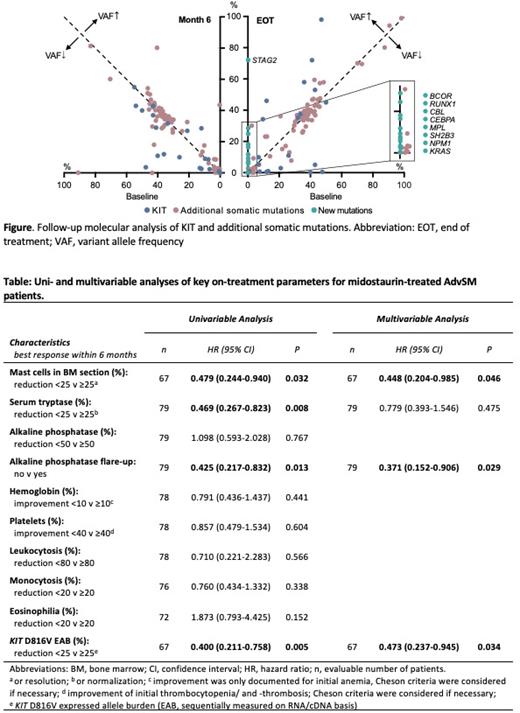
Results: Midostaurin was used in first-line (1L) or second and further lines (2L+; median 1 prior treatment line, range 0-4) in 63 (80%) and 16 (20%) patients, respectively. In 1L/2L+, median overall survival (OS) was 2.6/2.4 years. At baseline, the presence of additional somatic mutations in SRSF2 (n=36, 46%, HR 2.0 [1.2-3.6], P=0.014), ASXL1 (n=15, 19%, HR 2.0 [1.0-3.9], P=0.043), and EZH2 (n=6, 8%, HR 4.3 [1.7-11.3], P=0.003) significantly stratified patients according to their hazard ratio-weighted risk (median OS, 5.8 vs. 2.9 vs. 1.0 years; P<0.001). In 25 evaluable patients, the variant allele frequency in 72 additional somatic mutations remained stable (mean change 8%, absolute change >5% in 16/72 (22%) mutations). New mutations were identified in 9/25 (36%) patients during follow-up (Figure). Durable responses (complete, partial, clinical improvement) according to modified Valent criteria, IWG-MRT-ECNM criteria or Pure Pathological Response (PPR) criteria over a median duration between 12 and 24 months were achieved in 60%, 29% and 13% of patients, respectively. Within the first 12 months, duration of response was associated with an OS benefit ( P<0.001). An AP flare-up was observed in 22/79 (28%) patients, was paralleled by contemporaneous decrease of serum tryptase (median -35%, range -82 - 191%) and resulted into improved OS (median 5.8 vs. 2.2 years, P=0.007). Multivariable analysis identified the AP flare-up ( P=0.029), a ≥25% reduction of bone marrow (BM) mast cell (MC) infiltration ( P=0.046) and a ≥25% reduction of the KIT D816V expressed allele burden (EAB; P=0.034) at any time within the first 6 months as independent markers for improved OS (Table). Dynamic risk assessment of the independent parameters during follow-up allowed to abrogate a negative baseline risk-categorization. The initial dose was 200 mg/d (100 mg BID, including patients with a prespecified rapid dose escalation), 150 mg/d or 100 mg/d in 63/79 (80%), 2/79 (3%) and 14/79 (18%) patients, respectively. In the combined 150 mg and 100 mg cohorts, a dose increase to 200 mg/day was achieved in 9/16 (56%) patients. The 200 mg could be maintained at month 3, 6, 12, 24, and 36 in 50/63 (79%), 30/48 (63%), 23/34 (68%), 15/22 (68%) and 9/9 (100%) patients, respectively. Overall, 96 adverse events (AE) led to dose-adjustment (including temporary or permanent discontinuation). Hematologic and nonhematologic toxicities accounted for 24 (25%) and 36 (38%) of AEs with neutropenia (n=8, 8%) and nausea/emesis (n=22, 22%) being the most common AEs leading to dose-adjustment. Of note, the various dose levels had no impact on response or survival.
Conclusions: Baseline mutations in SRSF2, ASXL1 and EZH2 conferred resistance to midostaurin. The various dose levels had no impact on response or survival. The on-treatment parameters AP flare-up, and a ≥25% reduction of BM MC infiltration and of KIT D816V EAB abrogated a negative baseline risk-categorization. No new safety signals occurred.
Disclosures
Schwaab:GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Blueprint Medicines: Consultancy, Honoraria; Novartis Pharma: Honoraria. Reiter:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AOP Orphan Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal